Abstract
Background: Belantamab mafodotin is an anti-BCMA monoclonal antibody conjugated to a microtubule inhibitor that is approved for the treatment of relapsed refractory multiple myeloma in patients after 4 prior lines of therapy. It has shown efficacy in these late-relapse patients as a single agent, but has been associated with ocular toxicity, a unique adverse effect not seen with other anti-myeloma therapies. In addition to dry eyes, belantamab associated ocular toxicity can manifest as loss of visual acuity and/or keratopathy. Visual acuity is the eye's ability to distinguish shapes and details at a given distance and keratopathy refers to microcyst-like epithelial changes which may cause patients to experience irritation, tearing, foreign body sensation or blurred vision.
Methods: This was a single-center, retrospective, observational study assessing patients who received at least one dose of belantamab from June 2015 to June 2021 at Dana-Farber Cancer Institute (DFCI). We preformed a chart review to determine the incidence and severity of ocular toxicities with belantamab and the resulting management. This includes dose reductions, treatment delays, and therapy discontinuations that resulted from ocular toxicity. Secondary objectives include efficacy of belantamab regimens including response rate, duration of response, progression-free survival, and overall survival.
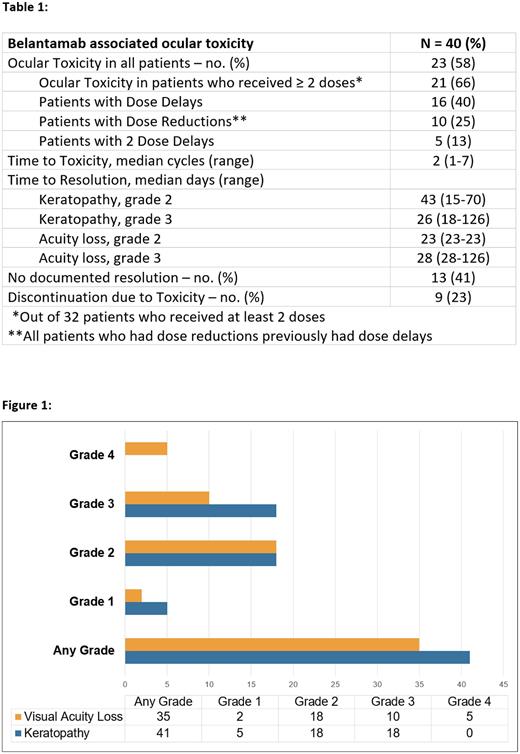
Results: Forty patients received at least one dose of belantamab at DFCI who were included in the analysis. High risk cytogenetics were found in 13 patients (33%) and 10 patients (25%) had extramedullary disease. History of ocular disease was found in 26 patients (65%). Thirty-eight patients (95%) received belantamab as monotherapy while 2 patients (5%) received as a combination regimen. Eight patients (20%) received one dose and the remaining 32 patients (80%) received at least 2 doses. Only 14 patients (35%) received ≥3 doses. Among patients that received at least 2 doses, 21 patients (66%) developed ocular toxicity (Figure 1). The median time to onset of ocular toxicity was 2 cycles (1 - 7) (Table 1). Dose delays occurred in 16 patients (40%) which were all due to ocular toxicity. Any grade keratopathy was found in 41% of patients and loss of visual acuity was found in 35% of patients. In total, 27 patients (67.5%) discontinued therapy, 18 patients (45%) due to progressive disease, 7 (17.5%) due to ocular toxicity, and 2 patients (5%) due to both ocular toxicity and progressive disease. Additionally, 6 patients (15%) died while on treatment. History of ocular disease did not corelate with belantamab related ocular toxicity. Single agent belantamab showed a meaningful response as the overall response rate (ORR) was 34% in this heavily pretreated population but due to frequent cases of ocular toxicity, there were treatment delays, dose reductions and progression of disease while therapy needed to be held.
Conclusions: This retrospective study confirms that belantamab mafodotin leads to a clinically meaningful response in a heavily pre-treated patient population. However, ocular toxicity affected two-thirds of patients who received at least 2 doses. This led to frequent treatment delays, dose reductions, and in some cases progression of disease. More long-term research is needed regarding the feasibility of alternative dosing strategies that could mitigate the ocular toxicity, such as prolonged intervals or cycle lengths, as well as combination with other anti-myeloma therapies.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal